Assassin’s tricks revealed in Nature
A team of Melbourne and London researchers have shown how a protein called perforin punches holes in, and kills, rogue cells in our bodies. Their discovery of the mechanism of this assassin is published today in the science journal Nature.
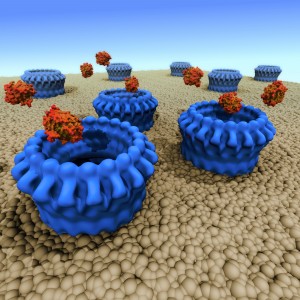
“Perforin is our body’s weapon of cleansing and death,” says project leader Prof James Whisstock from Monash University.
“It breaks into cells that have been hijacked by viruses or turned into cancer cells and allows toxic enzymes in, to destroy the cell from within. Without it our immune system can’t destroy these cells. Now we know how it works, we can start to fine tune it to fight cancer, malaria and diabetes,” he says.
The first observations that the human immune system could punch holes in target cells was made by the Nobel laureate Jules Bordet over 110 years ago. But how?
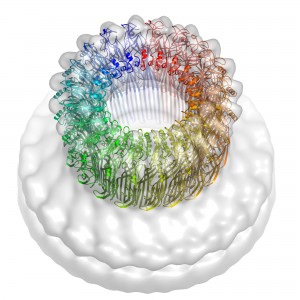
Researchers from Monash University and the Peter MacCallum Cancer Centre in Melbourne, and Birkbeck College in London collaborated on the ten-year study to unravel the molecular structure and function of perforin—the protein responsible. The structure was revealed with the help of the Australian Synchrotron, and with powerful electron microscopes at Birkbeck. Combining the detailed structure of a single perforin molecule with the electron microscopy reconstruction of a ring of perforins forming a hole in a model membrane reveals how this protein assembles to punch holes in cell membranes.
The new research has confirmed that the important parts of the perforin molecule are quite similar to those in toxins deployed by bacteria such as anthrax, listeria and streptococcus. “The molecular structure has survived for close to two billion years, we think,” says Prof Joe Trapani, head of the Cancer Immunology Program at Peter Mac.
“This work is a dramatic illustration of the importance of the synchrotron,” says Whisstock. “We simply couldn’t have done it without this wonderful facility.”

The weapon of death is a powerful molecule. If perforin isn’t working properly the body can’t fight infected cells. And there is evidence from mouse studies, says Trapani, that defective perforin leads to an upsurge in malignancy, particularly leukaemia.
Perforin is also the culprit when the wrong cells are marked for elimination, either in autoimmune disease conditions, such as early onset diabetes, or in tissue rejection following bone marrow transplantation.
So the researchers are now investigating ways to boost perforin for more effective cancer protection and therapy for acute diseases such as cerebral malaria. And with the help of a $1 million grant from the Wellcome Trust they are working on potential inhibitors to suppress perforin and counter tissue rejection.
The lead authors are Ruby Law from Monash University, Natalya Lukoyanova from Birkbeck College, London, and Ilia Voskoboinik from the Peter MacCallum Cancer Centre and the University of Melbourne. The project leaders are: Joe Trapani (Peter Mac), Helen Saibil (Birkbeck) and James Whisstock (Monash). The research was supported by the above institutions, the NHMRC, the ARC , the UK Biotechnology and Biological Sciences Research Council and the Wellcome Trust.
Feature story/backgrounder and photos available online at http://www.scienceinpublic.com/blog/embargoed/perforinbackgrounder
The Nature article is available online at http://www.nature.com/nature/journal/vaop/ncurrent/full/nature09518.html (you will need a subscription to access the full article)
For interviews contact:
James Whisstock, James.Whisstock@med.monash.edu.au
Joe Trapani, joe.trapani@petermac.org;
Helen Saibil, h.saibil@mail.cryst.bbk.ac.uk.





